Objective
At the end of the lesson, you should be able to describe pure substances, elements, and compounds.
Matter is anything that occupies space and mass. It can be classified based on its composition and properties.
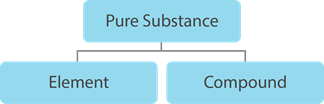
Pure Substance
- One of the classifications of matter.
- It is homogeneous.
- It has a definite composition and unique set of properties.
- The composition of a pure substance does not vary from one sample to another.
- Pure substances cannot be separated by physical means.
- It can be classified into two: elements and compounds.
Element
- It is the simplest form of pure substance.
- It is composed of only one kind of atom. Each atom of an element is unique to a given element.
- Elements cannot be broken into simpler substances either through physical or chemical means. However, elements can be transformed to other elements through nuclear reactions.
- An element can also exist either as a single atom or a molecule.
Examples
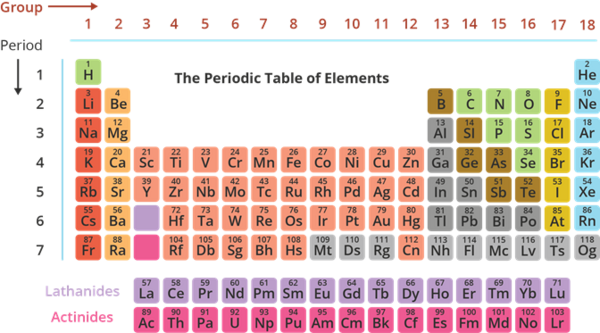
The Periodic Table of Elements
- It is a systematic and organized way of presenting elements.
- Currently, there are 118 known elements in which 94 elements are naturally occurring. The rest are synthetically made in the laboratory through nuclear processes.
- An element is represented by an element symbol which consists of one or two letters. In writing the element symbol, the first letter is written in upper case and the second letter is in lowercase.
- Columns in the periodic table are called groups (also families). Elements under the same group have the same chemical and physical properties. There are 18 groups in the periodic table.
- Rows in the periodic table are called periods. The periodic table has 7 periods.
Compound
- It is composed of two or more elements combined chemically. It also has a fixed proportion by mass.
- The formation of compounds follows the law of definite proportion. It states that regardless of the source, a given compound is always composed of the same kind of elements in the same proportion by mass.
- When two or more different elements combine to form a compound, the resulting compound has different properties compared to the individual elements.
- It can be decomposed into simpler substances by chemical means only. When decomposed, the resulting simpler substances also have different properties compared to the original compound.
- It is represented through a chemical formula. A chemical formula represents the elements and the number of elements present in a compound.
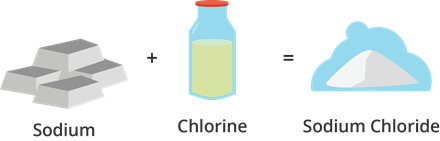
Example
Table salt is made up of sodium and chlorine atoms. Sodium is a very reactive metal which readily explode upon contact with water while chlorine is a poisonous gas. The combination of sodium and chlorine results to a useful compound known as table salt (or sodium chloride).
Key Points
- Matter is anything that occupies space and mass.
- A pure substance is homogeneous. It has a definite composition and unique set of properties.
- An element is composed of only one kind of atom. It is the simplest form of pure substance.
- Elements are presented in a systematic and organized way in a periodic table.
- A compound is a composed of two or more elements combined chemically and by fixed proportion by mass.

Comments
Post a Comment