Objectives
At the end of the lesson, you should be able to:
- classify solutions based on the amount of solute present; and
- define saturated, unsaturated and supersaturated solutions.
Solutions can also be classified based on the amount of solute dissolved in a given amount of solvent at a specific temperature.
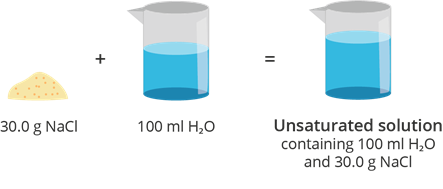
Unsaturated Solution
A solution in which the amount of solute is less than the solute’s solubility at a given volume and temperature is called an unsaturated solution.
Example
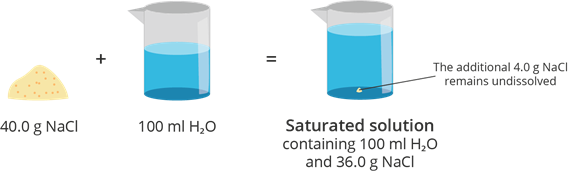
Saturated Solution
A solution in which the amount of solute is equal to the solute’s solubility at a given volume and temperature is called a saturated solution. It contains the maximum amount of solute that can be dissolved in a given amount of solvent and specific temperature.
Example
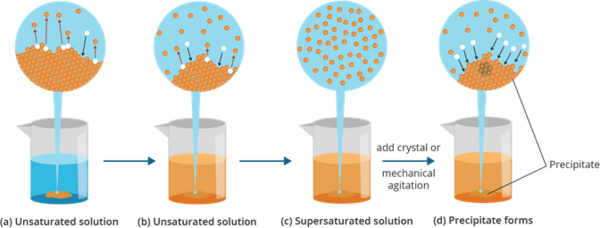
- This is usually done by dissolving a solute at a higher temperature, and subsequently cooling the solution.
- This state is an unstable state, which by slight agitation, some of the solute will come out of the solution, in a process called precipitation.
- Once precipitation occurs, the end result is a saturated solution.
Example
Key Points
- A solution in which the amount of solute is less than the solutes’ solubility is called an unsaturated solution.
- A solution in which the amount of solute is equal to the solute’s solubility is called a saturated solution.
- A solution in which the amount of solute greater than the solute’s is called a supersaturated solution.
Comments
Post a Comment