Objective
At the end of the lesson, you should be able to:
- discuss mixture and its type; and
- differentiate solutions, suspensions, and colloids.
Mixture
- It is composed of two or more pure substances combined through physical means in varying proportions.
- Each pure substance in a mixture retains its property.
- The components of a mixture can be separated by physical means depending on the states of the pure substances in a mixture.
- It can be classified into two namely homogeneous and heterogeneous mixtures.
Homogeneous Mixture
- It has uniform composition and properties.
- It has only one phase in which it is hard to determine the components of the mixture visually.
Example
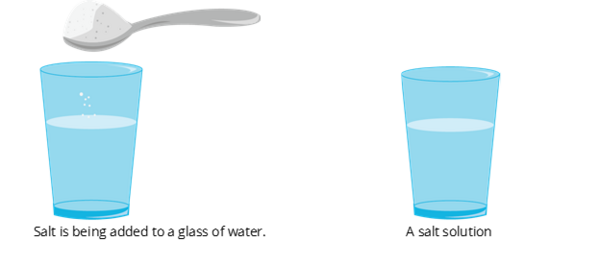
A salt solution is a homogeneous mixtures.
Heterogeneous Mixture
- It has varying composition and properties.
- It may have two or more phases since the individual substance is visually distinct
Example
An oil and water mixture is a heterogeneous mixture.
Solution, Suspension and Colloid
- Mixtures can also be classified based on particle size namely solution, suspension, and colloid.
- A solution is a homogeneous mixture while a suspension and a colloid are heterogeneous mixtures.
1. Solution
- It has two or more substances uniformly dispersed throughout the mixture. It forms a single phase
- It has the smallest particle size. Particles of a solution are invisible to the naked eye.
2. Suspension
- The components of a suspension separate over time due to the influence of gravity.
- It has the largest particle size compared to the other types of mixtures. The particles in a suspension are visible to the naked eye
3. Colloid
- The particles in a colloid only remain suspended in the medium when viewed using a microscope.
- It has an intermediate particle size compared to solutions and suspensions. The particles appear to be invisible to the naked eye.
Examples
- Solution: salt-water mixture and metal alloys.
- Suspension: soil-water mixture
- Colloid: milk and paint
Tyndall Effect
Colloidal particles exhibit Brownian movement and cause Tyndall effect.
- Brownian movement is the random movement of particles suspended in gas or liquid.
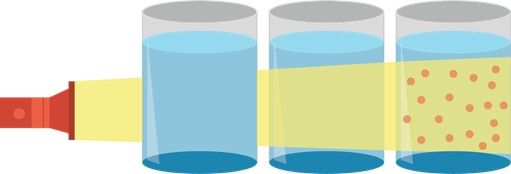
- This random motion of particles causes the scattering of light or Tyndall effect. Tyndall effect is usually demonstrated by passing a ray of light through a sample mixture.
- Tyndall effect could be used to identify the mixture as solution, colloid, or suspension.
- When light passes through a given mixture, the mixture is a solution.
- When light does not pass through a given mixture, the mixture is a suspension.
- When light passes through a given mixture and the light scatters, the mixture is a colloid.
Key Points
- A mixture is composed of two or more pure substances combined through physical means in varying proportions. Its components retain its property and can be separated by physical means.
- Mixture can be classified into two.
- A homogeneous mixture has uniform composition and properties and has only one phase.
- A heterogeneous mixture has varying composition and properties. It may have two or more phases.
- Mixtures can also be classified based on particle size namely solution, suspension, and colloid. A solution is a homogeneous mixture while a suspension and a colloid are heterogeneous mixture.
- A solution has two or more substances uniformly dispersed throughout the mixture. It has the smallest particle size.
- A suspension has the largest particle size. The components of a suspension separate over time due to the influence of gravity.
- A colloid has an intermediate particle size. The particles in a colloid remain suspended in the medium. However, it appears to be invisible to the naked eye.
- Colloidal particles exhibit Brownian movement and cause Tyndall effect.
- Brownian movement is the random movement of particles suspended in gas or liquid.
- Tyndall effect is the scattering of light.


Comments
Post a Comment