Objectives
At the end of the lesson, you should be able to
- define solute and solvent; and
- classify different types of solutions based on the nature of solute and solvent.
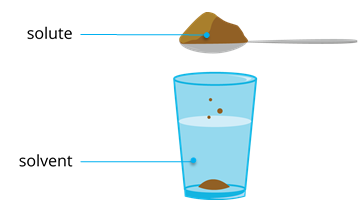
A solution is simply a homogeneous mixture of two or more pure substances. It consists of a solute and a solvent.
- The more abundant substance is the solvent. It is the dissolving medium.
- The substance present in less amount is the solute. It is the substance being dissolved.
Example
In a solid-liquid solution such as coffee powder dissolved in water. The coffee powder is the solute while water is the solvent.
Types of Solutions
Solutions are classified based on the states of the solute and solvent.
1. Liquid solutions are solutions where the solvent is a liquid. It is the most common type of solution.
1. Solid solutions are solutions where the solvent is a solid.
1. Gaseous solutions are solutions where the solvent is a gas.
Example
The mortar and pestle above is made of brass. Brass, an alloy is an example of a solution.
Examples
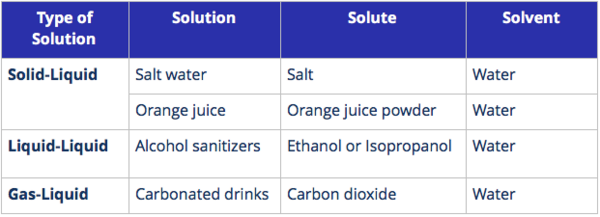
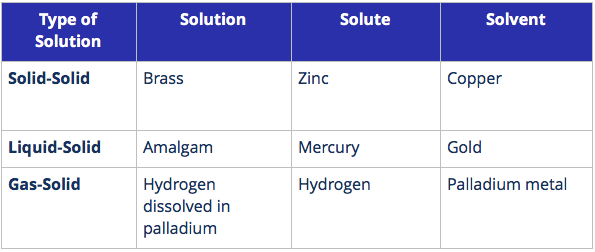
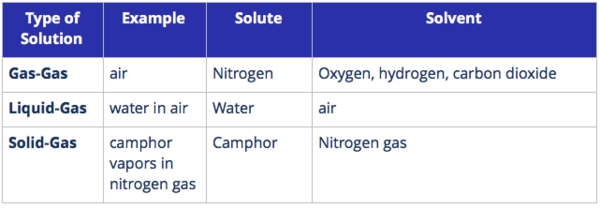
Key Points
- A solution is simply a homogeneous mixture of two or more pure substances.
- It consists of a solute and a solvent.
- The more abundant substance is the solvent. It is also defined as the dissolving medium.
- The substance present in less amount is the solute. It is also defined as the substance being dissolved.
- Solutions are classified based on the states of the solute and solvent. They are classified as liquid solutions, solid solutions, and gaseous solutions.

Comments
Post a Comment