Objectives
At the end of the lesson, you should be able to:
- distinguish mixtures from pure substances based on a set of properties; and
- identify various method in separating the components of a mixture.
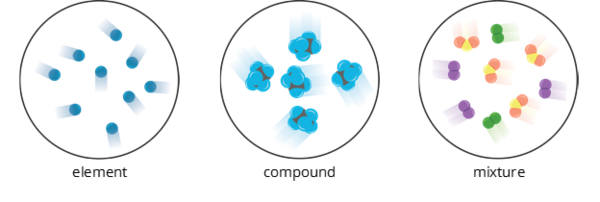
Pure Substance
- It is always homogeneous.
- It has uniform properties and definite composition.
- It has a constant composition and combined chemically.
- It cannot be separated into its components by chemical means.
- It is either classified as an element or a compound.
Mixture
- It has variable compositions made up of pure substances.
- The pure substances that make up a mixture are combined through physical means.
- Each pure substance in a mixture retains its properties.
- It can be classified as solution (homogeneous), and colloids and suspension (heterogeneous).
Distinguishing Pure Substances and Mixtures
1. Atomic Level
- An element is composed of only one kind of atom.
- A compound is composed of two or more elements combined chemically.
- A mixture is made up of two or more pure substances.
2. Composition
- The composition of pure substances does not vary from one sample to another. It is uniform in any sample of a pure substance.
- The composition of mixtures varies and the property of each constituent pure substance is retained.
3. Manner of Separation of Components
- Pure substances cannot be separated by physical means.
- Mixtures can be separated by physical means.
Separating Mixtures
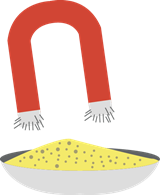
1. Physical Separation
- It separates the components of varying sizes using a spoon or any scooping material or by picking.
- The use of magnet can be done to separate the metallic materials from non-metallic materials.
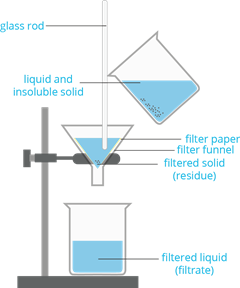
2. Filtration
- It is a method to separate an insoluble solid from a liquid mixture using a semipermeable membrane like filter paper.
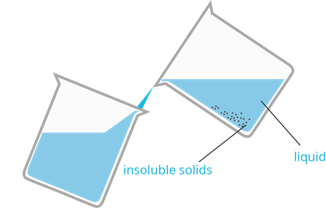
3. Decantation
- It is a method used to separate large particles of insoluble solid from the liquid mixture.
- The mixture is allowed to stand to separate the liquid layer from the solids that have settled.
Separating Mixtures
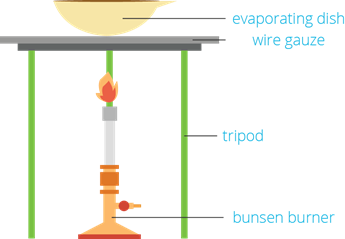
4. Evaporation
- It is a method to separate the soluble solid from the liquid component of the solution by evaporating the liquid substance.
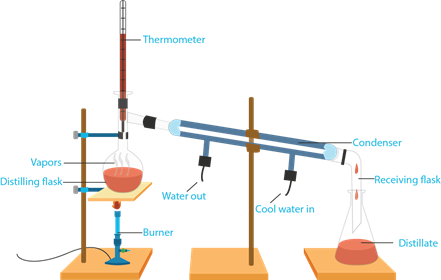
5. Distillation
- It is a method to the components of a mixture of liquids based on the difference in boiling point.
6. Centrifugation
- It is a method used to separate the components based on the difference in density.
Key Points
- At the atomic level, an element is composed of only one kind of atom; a compound is composed of two or more elements combined chemically; and a mixture is made up of two or more pure substances.
- The composition of pure substances does not vary from one sample to another. The composition of mixtures varies.
- Pure substances cannot be separated by physical means. Mixtures can be separated by physical means.
- Mixtures can be separated by physical separation, filtration, decantation, evaporation, distillation, and centrifugation.

Comments
Post a Comment