Objective
At the end of the lesson, you should be able to distinguish elements from compounds based on a set of properties.
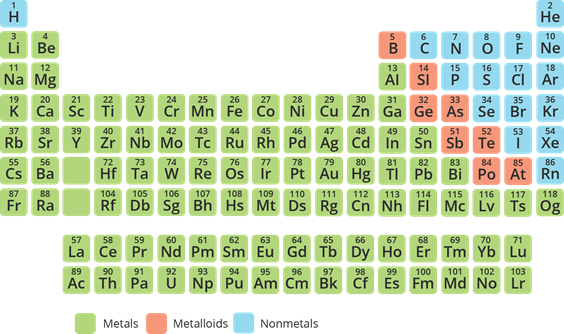
Elements and compounds are classes under pure substances.
Element
- It is the simplest form of pure substance.
- It is composed of only one kind of atom.
- It could be classified into three: metals, nonmetals, and metalloids.
Compound
- It is composed of two or more elements combined chemically in a fixed proportion by mass.
- It can be classified in terms of properties and elements present.
Compounds: Acids and Bases
Acids
- taste bitter
- turns blue litmus paper to red
- reacts with metals to produce hydrogen gas
Bases
- taste sour
- feel slippery
- turns red litmus paper to red
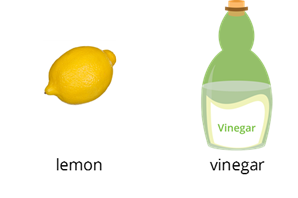
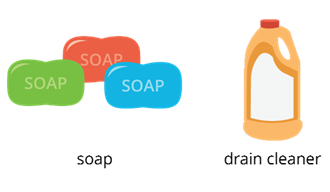
Examples
Examples of acids are lemon and vinegar.
Examples of bases are soap and drain cleaner.
Compounds: Organic and Inorganic
Organic compounds are compounds containing carbon. However, there are carbon-containing compounds which are considered as inorganic such as carbon monoxide, carbon dioxide, cyanide, carbonate, bicarbonate and carbon disulfide.
Examples
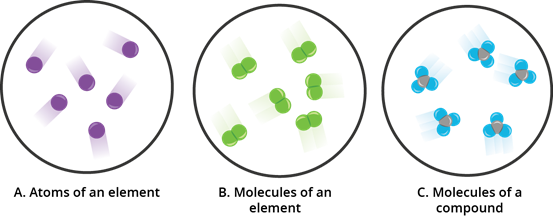
- Elements exist in single atoms.
- Some elements exist in molecules. It consists of more than one atom of the similar type.
- A compound is composed of two or more kinds of atom.
Key Points
- An element is composed only of one kind of atom.
- Elements can be classified into three: metals, nonmetals, and metalloids.
- A compound is composed of two or more elements combined chemically in a fixed proportion by mass.
- Compounds can be classified in terms of properties (acids and bases), and elements present (organic and inorganic).

Comments
Post a Comment